Transcranial Magnetic Stimulation (TMS)
Discover the Transformative Power of Transcranial Magnetic Stimulation
When you’re struggling with depression, it can be hard to see the light at the end of the tunnel. But now more than ever, there is one – TMS.
If you’ve tried more than two antidepressants without relief of your depression symptoms, or are experiencing uncomfortable side effects on top of your usual symptoms, then Transcranial Magnetic Stimulation (TMS) therapy might be the solution. This innovative and alternative depression treatment option is FDA approved and provides positive and long-term results with little to no side effects.
Please don’t lose hope. Give TMS therapy a try.
What is TMS Therapy?
TMS stands for “Transcranial Magnetic Stimulation.” This revolutionary depression treatment approach was FDA approved in 2008 as a safe, non-invasive, non-medication treatment for clinical depression. TMS therapy is designed to help people with mental health conditions who have not found success with medications and/or antidepressants, utilizing magnetic fields to stimulate the specific part of the brain known to control mood.
TMS treatment has extremely limited side effects, especially when compared to traditional medications and antidepressants, so it is also a treatment option for people who may be suffering from side effects while trying to experience relief from their condition.
What does TMS treat?
Since its FDA approval as a depression treatment in 2008, further studies have shown that TMS has the potential to treat a wide range of conditions, many of which can be debilitating. Although the Mindful Health Solutions team focuses primarily on alleviating the symptoms of treatment-resistant depression, our clinics also provide treatment for other conditions, like:
- Anxiety
- Autism in adults
- Bipolar disorder
- Body dysmorphia
- Certain types of chronic pain
- Memory disorders
- Migraines
- Mild cognitive impairment
- Mild dementia
- Multiple sclerosis
- Obsessive-Compulsive Disorder (OCD)
- Parkinson’s disease
- PTSD
- Smoking cessation
There is also evidence TMS treatment may help in recovery from stroke. Research and studies on the impact of TMS therapy are continuing, and we at Mindful Health Solutions are excited to be at the forefront of this revolutionary treatment.
How does TMS work?
Individuals experiencing depression are shown to have reduced electrical activity in certain areas of the brain. TMS treatment works by delivering highly targeted, localized electromagnetic pulses to those areas, including the prefrontal cortex, which is the area of your brain that regulates mood. These electromagnetic pulses stimulate the affected neurons, which in turn releases neurotransmitters and hormones such as serotonin and dopamine. These neurotransmitters work to relieve symptoms of depression. Once these neurons are stimulated by TMS, they continue to release these neurotransmitters for a longer period of time compared to other treatments like antidepressants.

Patients who failed 2 antidepressants are 2x more likely to achieve remission with TMS.
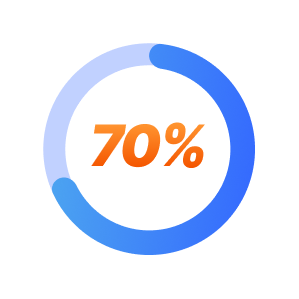
TMS is effective in approximately 70% of patients.

Patients who receive TMS versus conventional medication are more likely to recover from depression at their 12 month follow-up.

What does a treatment session look like?
During a TMS treatment, the patient sits in a comfortable chair while the TMS coil is properly set in place on their head. (While this may sound intimidating, it really isn’t!) After the headpiece is in place, electromagnetic pulses are sent to the prefrontal cortex, which is the area of the brain responsible for mood.
While the pulses are being sent to the brain, patients can watch TV, listen to music, or talk with our staff. If they want and are comfortable, they can also have a friend or family member in the room with them during their TMS treatment session.
Each treatment lasts about 20-30 minutes. The full course of treatment will typically be five days a week for four to six weeks. Generally, patients will feel just like their normal selves after their treatment sessions. They will be able to drive and go back to their regular activities. Some people report feeling a little tired or having a mild headache, but that usually goes away after the first week or two. Other people report more energy and better focus, as well as the ability to ignore obsessive, anxious thoughts after treatment.
What Our Patients Are Saying about the Power of TMS Therapy
“TMS gave me a life back.”
Christy, TMS Patient
Hear from a Mindful Health Solutions patient on how Transcranial Magnetic Stimulation improved her mental well-being and transformed her outlook on life.
Your Path to Transcranial Magnetic Stimulation Treatment
In order to qualify for TMS treatments, you have to have tried at least two medication management plans and either not experienced relief of your symptoms or experienced uncomfortable side effects from them. If that is the case, your provider will talk to you about integrating TMS therapy into your customized treatment plan.
It is important to understand that mental health treatment plans are not a one-size-fits-all situation. Because of this situation, depression treatment plans for Transcranial Magnetic Stimulation may take longer or require different treatment option combinations to work best for certain patients. It is important for patients to communicate clearly with their provider about how their treatment plan is working. Collaboration between patient and provider can greatly impact the results of a patient’s wellness journey.



We’re Paving the Way in Depression Care with TMS Therapy
Mindful Health Solutions is the nation’s leader provider of TMS therapy to patients like you. In fact, we founded one of the first TMS-focused clinics in the US and have deep expertise in the field of non-invasive brain stimulation. Patients at Mindful Health Solutions have access to true thought leaders in the field focused on bringing academic rigor and years of experience with novel therapeutics such as Transcranial Magnetic Stimulation to an integrated treatment strategy.
If you are considering TMS treatment, you can rest assured that you will get the most evidence-based, safe, and effective treatment possible with us at Mindful Health Solutions.

Why Choose Mindful Health Solutions?
Sadly, depression is an all-too-common condition that impacts millions of people in the United States alone. We want to bring that number down one person at a time, starting with you.
Overall, we understand the importance of patient-centered care and taking a true individualized approach. Patients respond well to the dynamic menu of treatments we offer, which includes traditional medication management as well as cutting-edge alternatives such as Transcranial Magnetic Stimulation (TMS), Spravato (Esketamine), and Ketamine IV Therapy.
Mindful Health Solutions also wants to make treatment comfortable and accessible. We offer telepsychiatry if patients are physically unable to come into the office or prefer a virtual appointment. This option allows patients to get care from wherever they are in the state.
Insurance
We are in-network with Kaiser, Medicare, and all major commercial insurance plans.
Frequently Asked Questions
How does TMS work?
Depression results when neurons are underactive in the area of the brain that regulates mood. TMS stimulates these neurons, returning them to normal function, thus alleviating depression symptoms. Over time, TMS encourages the brain to regulate activity on its own.
Still wondering, “How does TMS work?” Reach out today for a consultation!
How successful is TMS?
Most patients with treatment-resistant depression respond favorably to TMS, and many achieve full remission even after years of illness. Many patients maintain their results well over a year after treatment, though some may require periodic follow-up sessions. Medication and psychotherapy are often more effective after a course of TMS and may help maintain the long-term therapeutic benefit.
How is Transcranial Magnetic Stimulation administered?
TMS is a clinic-based, outpatient procedure. No anesthesia is used, so patients can drive themselves to and from their appointments and resume their normal activities right away. A typical course of treatment consists of 30 to 36 sessions administered over a 4-6 week time period. Each session lasts around 20 minutes.
How is TMS better than medications?
While medications are an invaluable tool in treating depression, they don’t work for everyone. If you have tried more than one antidepressant with no relief, or have experienced uncomfortable side effects, TMS is a proven option for you. TMS only targets certain areas of the brain that impact mood regulation, meaning it doesn’t cause side effects associated with medication. Most importantly, patients who failed two antidepressants are twice as likely to achieve remission with TMS, meaning their symptoms completely go away.
What are the side effects of TMS?
TMS treatments do not hurt during or after each session, and there are very few side effects. While some patients find the headpiece uncomfortable and may experience mild headaches during the first few treatments, these symptoms typically subside within two weeks. Most side effects, such as headaches, fatigue, lightheadedness, scalp discomfort, and facial muscle twitching or tingling, are mild and short-lasting. Serious side effects are rare, and our expert providers use their professional knowledge to minimize any risks.
What does TMS treat?
Since its FDA approval as a depression treatment in 2008, further studies have shown that TMS has the potential to treat a wide range of conditions, many of which can be debilitating. Although the Mindful Health Solutions team focuses primarily on alleviating the symptoms of treatment-resistant depression, our clinics also provide treatment for other conditions like anxiety, autism in adults, bipolar disorder, body dysmorphia, certain types of chronic pain, memory disorders, migraines, mild cognitive impairment, mild dementia, multiple sclerosis, obsessive-compulsive disorder (OCD), Parkinson’s disease, PTSD, and smoking cessation. There is also evidence that TMS may help in recovery from stroke. Research and studies on the impact of TMS are continuing, and we at Mindful Health Solutions are excited to be at the forefront of this innovative therapy.
